Hand Sanitizer Intermediates
OVERVIEW
Aquaserv offers an intermediate for use in benzalkonium chloride-based hand sanitizers. Our product is geared towards providing our customers with sustainable, technologically advanced, and economical solutions to meet their needs. Listed below is our 50% benzalkonium chloride (BZK) that we currently offer to be used in the production of hand sanitizers. If you should have any questions pertaining to this product, please ask us as we are frequently adding new products and would like the opportunity to collaborate with you to develop the most effective and environmentally responsible solutions to overcome your disinfecting and sanitizing challenges.


AQ-BZK, 50 %
AQ-BZK, 50% is a 50% active benzalkonium chloride solution in water that is certified to meet United States Pharmacopeia (USP) and National Formulary (NF) requirements. It is produced under appropriate cGMP controls for active pharmaceutical ingredients. AQ-BZK, 50% meets FDA guidelines for use as a cosmetic ingredient broad spectrum preservative and active ingredient in OTC topical antimicrobials/antiseptics such as antibacterial hand soaps and sanitizers.
Benzalkonium chloride-based hand sanitizers have distinct advantages over gelled alcohol hand sanitizers. While both product forms are fast acting and allow for use without water or towels, benzalkonium chloride-based products are non-flammable, less drying to skin, and will not stain clothing. Published studies report that benzalkonium chloride based hand sanitizers demonstrated greater sustained degerming activity than gelled alcohol gel hand sanitizers that actually became less effective with repeated use and made the skin dirtier, not cleaner, due to removal of protective natural skin oils and entrapment of dead skin cells by the polymer thickeners used in the gelled alcohol products (AORN Journal, (68 August 1998), p. 239-251).
Benefits:
- Broad spectrum efficacy
- High, stable foam properties
- Alcohol-free
- Use as a cosmetic ingredient broad
spectrum preservative and active Ingredient
in topical antimicrobials
- Produced under appropriate cGMP controls
for active pharmaceutical ingredients - Certified to meet United States
Pharmacopeia (USP) and National
Formulary (NF) requirements for
benzalkonium chloride solution.
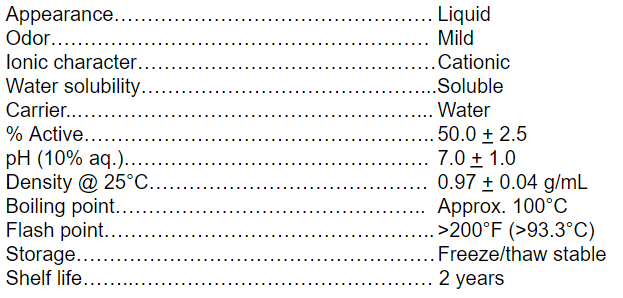
Typical Properties

AQ-HS-100
AQ-HS-100 is a 1% active benzalkonium chloride-based foaming, antibacterial hand sanitizer 10X concentrate produced under appropriate cGMP controls in an FDA registered establishment (Identification No.: 3010203410) for active pharmaceutical ingredients. AQ-HS-100 conforms to FDA guidelines for use as an OTC topical antimicrobial “leave-on” hand sanitizer.
Benzalkonium chloride is listed in the antiseptic monograph as category III for safety and efficacy. This category allows benzalkonium chloride-based products to be marketed in use patterns that fall within the monograph as long as the formulations are manufactured under Good Manufacturing Practices (GMP’s) and conform to the percentage range in the monograph of 0.1-0.13% for benzalkonium chloride.
AQ-HS-100 produces a fast drying, non-sticky foam that contains unique non-drying, conditioning and moisturizing ingredients, and leaves the skin with a soft, refreshing and silky after-feel. AQ-HS-100 does not contain PEGs, polymer thickeners, silicones, or ingredients additionally recognized as active ingredients.
Benefits:
- Fast drying
- Broad spectrum efficacy
- High, stable foam properties
- Alcohol-free
- Readily dilutable 10X concentrate
- Contains no PEGs, polymer thickeners or silicones
- Contains moisturizing/conditioning ingredients to provide a silky after-feel
Typical Properties:


AQ-HS-100G
AQ-HS-100G is a 1% active benzalkonium chloride-based antibacterial hand sanitizer gel 10X concentrate produced under appropriate cGMP controls in an FDA registered establishment (Identification No.: 3010203410) for active pharmaceutical ingredients. AQ-HS-100G conforms to FDA guidelines for use as an OTC topical antimicrobial “leave-on” hand sanitizer.
Benzalkonium chloride is listed in the antiseptic monograph as category III for safety and efficacy. This category allows benzalkonium chloride-based products to be marketed in use patterns that fall within the monograph as long as the formulations are manufactured under Good Manufacturing Practices (GMP’s) and conform to the percentage range in the monograph of 0.1-0.13% for benzalkonium chloride.
AQ-HS-100G produces a clear, non-alcohol gel that is an effective alternative to thickened-alcohol based hand sanitizer products. AQ-HS-100G formulations meet the FDA drug claim “reduces bacteria on the skin,” leaving the skin with a soft, refreshing, and silky after-feel. Its unique skin-friendly pH gel system does not leave behind a sticky-film that can trap dead skin cells and bacteria, resulting in healthier skin.
Benefits:
- Fast drying
- Broad spectrum efficacy
- Alcohol-free, non-flammable
- Noncomedogenic
- Readily dilutable 10X concentrate
- Carbomer-free
- Sheer thinning gel at skin-friendly pH
- Contains moisturizing/conditioning
ingredients to provide a silky after-feel
Typical Properties:


AQ-AF-120
AQ-AF-120 is a 20% active, nonionic, short-chain fluoroacrylate polymer and fluoroaliphatic alcohol blend optimized for foaming aqueous blends of alcohols and polar organic solvents. When formulated into aqueous alcohol-based formulations, AQ-AF-120 generates, rich, stable foam through aeration, agitation, or combined with an inert gas to form a froth or foam while leaving a soft, refreshing, non-sticky after-feel. Consumers appreciate the sensory appeal of a fast-drying foam over a sticky gel as well as an effective product without the polymer thickeners that can attract and promote the growth of bacteria on the skin.
Besides use in alcohol-based hand sanitizers, AQ-AF-120 can be used in cosmetic formulations ranging from foamable shampoos and personal cleansers to make-up and nail polish removers. Ingredients typically requiring complicated emulsion systems can be foamed utilizing AQ-AF-120 such as sunscreen and insect repellent foams, analgesic rubs, antiperspirants, and pet grooming products to name a few.
INCI Names:
Acrylates / perfluorohexylethyl methacrylate copolymer
Perfluorohexylethyl alcohol
Benefits:
- Readily dilutes in aqueous alcohol and
polar organic solvents - Delivers thick, stable foam
- Leaves a soft, refreshing, non-sticky
after-feel
- Dimethicone and silicone free
- Fast drying
- Short-Chain fluorochemical technology (meets
the goal of the US EPA 2010/2015 PFOA
Stewardship Program)
Typical Properties:


Get in Touch With Us
Contact us if you do not see a particular chemistry you are interested in
as we are frequently adding new products. We would like to collaborate with you
to develop the most effective and environmentally responsible solution to overcome any challenges.

